PSIG
Famous Member
May as well drag several things related out onto the carpet at once.  This is a conversation sharing ideas, data, and experience as interpretations, opinions or hypothesis. Most of us have learned that nothing is ever set in stone, and many factors affect outcomes. Conclusions should not be drawn from a single data point or event. While an idea may sound good, it may not be the actual cause or solution. Therefore, there may be disagreement or challenges, but 'correction', 'right' or 'wrong' is not possible with another opinion.
This is a conversation sharing ideas, data, and experience as interpretations, opinions or hypothesis. Most of us have learned that nothing is ever set in stone, and many factors affect outcomes. Conclusions should not be drawn from a single data point or event. While an idea may sound good, it may not be the actual cause or solution. Therefore, there may be disagreement or challenges, but 'correction', 'right' or 'wrong' is not possible with another opinion.
This is recognized by EFI systems and why they use intake air, manifold air and coolant temperature sensors to correct and maintain the tune through temperature variation. That's why they are there and the benefits they bring (within limits). Also, why maintaining thermostat control and minimum temperature variation is a top cooling system goal. Cooling system function affects a lot!
Geek stuff - skip if you don't care: I'll note here that the Reid Vapor Pressure (RVP) of ethanol has a temperature transition, where the curve is higher above 61°F (16°C). This is why high percentages of ethanol are harder to start in cold weather, but once temperatures rise it has high RVP. In-fact, pump gas blends are restricted as the RVP is not suppressed sufficiently at higher temperatures with butanol/butane or alkane additives, etc. From EPA reference:
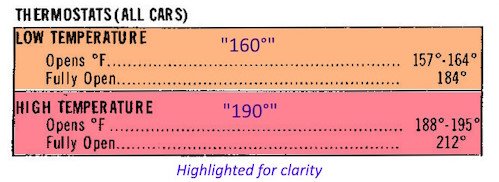
 Below is a copy of the Ford graphic, highlighted for clarity between the two alternate temperatures:
Below is a copy of the Ford graphic, highlighted for clarity between the two alternate temperatures:
Actually they are, as only vapor can be burned. Fuel droplets - no matter how small - cannot burn, no more than wood burns but its smoke vapor does. Do not confuse vapor to burn with wet vs vapor manifold fuel distribution. Also notice that whether pulled through a carb or injected as wet fuel at the head, both methods vaporize the fuel for combustion — meaning the fuel vaporization for combustion is a separate conversation from wet or vaporized fuel movement in the intake manifold.Interesting, please expound on the vaporization of gas/ ethonal, I remember that carbs atomized fuel ( same with injectors ). Engines aren’t designed to run on vapor
These issues are primarily tune-related, and any intentional temperature range may be tuned for relative peak power or economy. I have seen this myself when temperatures rose due to crap on the radiator, and once removed it returned to normal power. If the tune is a rich or lean, the effects will be exaggerated by temperature variation.drag-200stang said:Oddly my high school 200 full carb bore sized tri-power ran faster at the track when run at 180, colder would run slower times.
63 Sprint said:This was experienced when the thermostat went bad on the 300. Poor fuel economy and performance was the end result.
This is recognized by EFI systems and why they use intake air, manifold air and coolant temperature sensors to correct and maintain the tune through temperature variation. That's why they are there and the benefits they bring (within limits). Also, why maintaining thermostat control and minimum temperature variation is a top cooling system goal. Cooling system function affects a lot!
The primary benefit to lower coolant temperatures are the under-hood, where the fuel supply lines and carb remain cooler in the wash from the hot radiator blown by the fan. As the manifold is already hot from the exhaust barbecue it's over (the thermostat does not affect that heating significantly), it's also trying to heat the carb above it (where the percolation issues stem). The thermostat has little effect on the manifold temperatures, and does not affect fuel vaporization significantly in the manifold. Conversely, the fuel temperature has a much larger effect on power.it is very difficult in my area to find no Ethanol fuel . So how does Ethanol fuel effect thermostat temp choices ?
Geek stuff - skip if you don't care: I'll note here that the Reid Vapor Pressure (RVP) of ethanol has a temperature transition, where the curve is higher above 61°F (16°C). This is why high percentages of ethanol are harder to start in cold weather, but once temperatures rise it has high RVP. In-fact, pump gas blends are restricted as the RVP is not suppressed sufficiently at higher temperatures with butanol/butane or alkane additives, etc. From EPA reference:
Yeah, it vaporizes too easily when warmer. The rest of the year, it is formulated to hold the same RVP as straight gasoline at RVP 9.0, and should vaporize the same as a blend. Use care not to compare high percentages or straight ethanol vs gasoline numbers. They change fuel character and are not comparable. Yes, there are secondary effects such as cumulative water absorption that can affect vaporization, but are not within this scope.kbracmort@crs.loc.gov said:At present, E15 cannot be sold during summer months because it does not meet the Reid Vapor Pressure (RVP) requirements for the summer ozone season (generally June 1-September 15).
Yeah, it's surprising how few have looked at that part of a manual, but then again most do not reference the FSMs, and the graphic is not clear. The assumption is that it should operate properly at either temperature. This is not inclusive of other uses, such as Ford marine engines using 140°F thermostats.Good to know that the 160 is listed in the 3.3 service manuals .

True! But IMO that's not the factor involved here. My 2¢ - as mentioned above, the manifold vaporization is little affected by the thermostat temperature. Vaporization is inclusive, based on the formulated RVP, which for E0 and E10 blend is equivalent at RVP 9. There is no fuel-based vaporization issue to 'fix', and is not a unique issue to the Ford six. The F6 has its own specific problems with manifold and runner design and their fuel distribution related to wet-flow or vaporization, but they are little related to limited ethanol in pump gas from the data, nor across many vehicle types and engines for real-world comparisons. We can solve for many shortcomings, but from everything I've seen the fuel characteristics between E0 and E10 are not a large factor in the thermostat conversation.The vaporization point of ethanol and gas are totally different. You need more heat than 160 degrees F in order for both the ethanol and gasoline to vaporize properly.
Last edited:
